VALVULAR PLUG III
STRUCTURAL INTERVENTIONS
Paravalvular leaks (PVLs) are a common and challenging problem around the world.1 The Amplatzer™ Valvular Plug III is specially designed to provide an effective solution to this key issue, improving quality of life and longevity for an increasing number of patients.2–5
BUILT ON THE EXTENSIVE AMPLATZER™ LEGACY OF SAFETY AND EFFICACY
- Pioneered transcatheter cardiovascular and peripheral vascular occlusion
- Over 1.25 million Amplatzer™ devices implanted worldwide9
- More than 20 years of clinical experience and global leadership
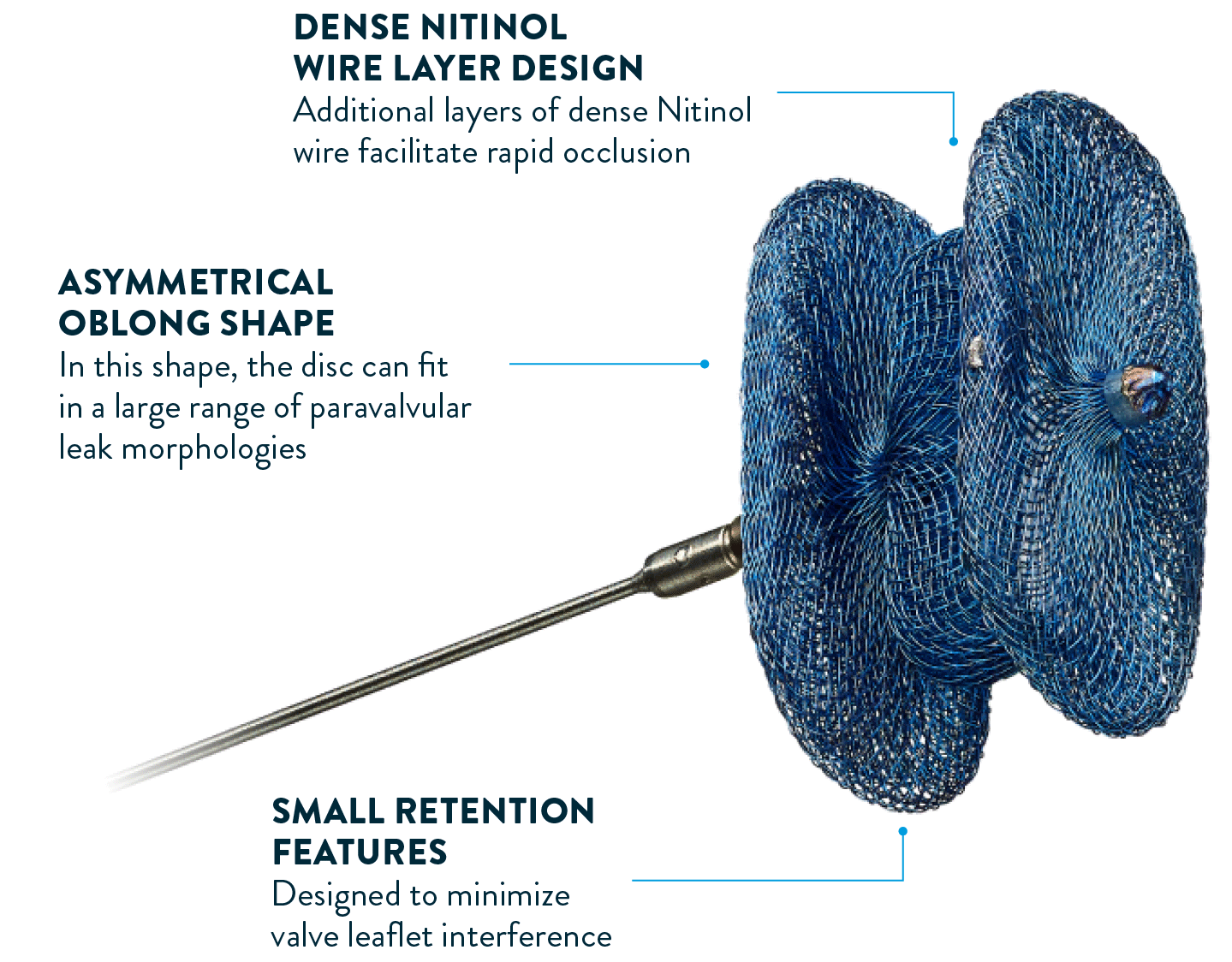
EVERY DETAIL IS DESIGNED
FOR SUCCESSFUL PVL CLOSURE
The Amplatzer™ Valvular Plug III has been specially designed to offer patients an effective solution to PVL. This oblong, self-expanding device is made from braided Nitinol wires with shape memory characteristics, making it well suited to PVL closure.2,6,7,9,10
To close PVL with different morphologies, the Amplatzer™ Valvular Plug III comes in a range of 9 sizes and has a flexible Nitinol waist, ensuring an excellent device-to-patient fit.
Connext
Live
Hear from the most respected KOLs in cardiovascular and stroke prevention, collaborate on best practice and be part of the CONNEXT community.
HUB
- Rama-Merchan J, Arribas-Jimenez A, et al. Prevalence and severity of paravalvular regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) echocardiography study. Rev Esp Cardiol. 2014;67:593-596.
- Ruiz CE, Hahn RT, Berrebi A, et al. Clinical trial principles and endpoint definitions for paravalvular leaks in surgical prosthesis: an expert statement. J Am Coll Cardiol. 2017;69(16):2067-2087. doi.org/10.1016/j.jacc.2017.02.038.
- Calvert PA, Northridge DB, Malik IS, et al. Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation. 2016;134(13):934-944. doi.org/10.1161/CIRCULATIONAHA.116.022684.
- García E, Arzamendi D, Jimenez-Quevedo P, et al. Outcomes and predictors of success and complications for paravalvular leak closure: an analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention. 2017;12(16):1962-1968. https://doi.org/10.4244/EIJ-D-16-00581.
- Davidavicius G, Rucinskas K, Drasutiene A, et al. Hybrid approach for transcatheter paravalvular leak closure of mitral prosthesis in high-risk patients through transapical access. J Thorac Cardiovasc Surg. 2014;148(5):1965-1969. doi.org/10.1016/j.jtcvs.2014.05.001.
- Smolka G, Pysz P, Jasinski M, et al. Multiplug paravalvular leak closure using Amplatzer Vascular Plugs III: a prospective registry. Catheter Cardiovasc Interv. 2016;87(3):478-487. doi.org/10.1002/ccd.25992.
- Yildirim A, Goktekin O, Gorgulu S, et al. A new specific device in transcatheter prosthetic paravalvular leak closure: a prospective two-center trial. Catheter Cardiovasc Interv. 2016;88(4):618-624. doi.org/10.1002/ccd.26439.
- Angulo-Llanos R, Sarnago-Cebada F, Rivera AR, et al. Two-year follow up after surgical versus percutaneous paravalvular leak closure: a non-randomized analysis. Catheter Cardiovasc Interv. 2016;88(4):626-634. doi.org/10.1002/ccd.26459.
- Data on File at Abbott.
- Sánchez-Recalde A, Moreno R, Galeote G, et al. Immediate and midterm clinical course after percutaneous closure of paravalvular leakage. Rev Esp Cardiol (Engl Ed). 2014;67(8):615-623. doi.org/10.1016/j.rec.2014.01.031.





