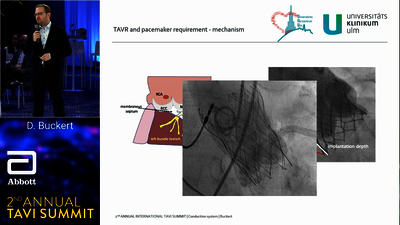
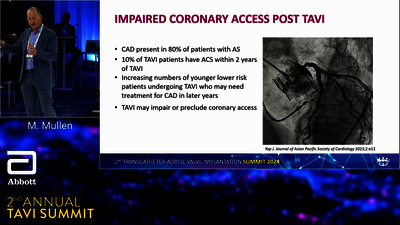
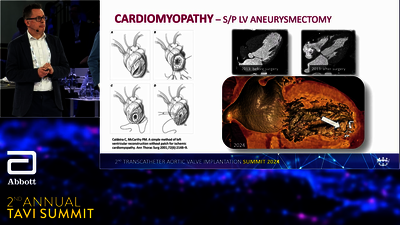
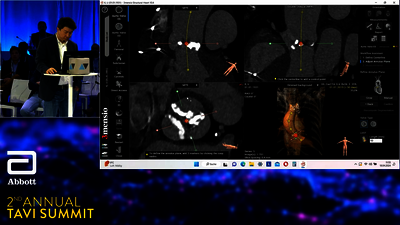
TAVI SYSTEM
TAVI
Stable delivery. Remarkable performance. Future ready.
ADVANCING THE FOREFRONT OF TAVI INNOVATION
The Navitor Transcatheter Aortic Valve Implantation (TAVI) System is at the cutting edge of minimally invasive heart valve replacement. Designed for stable delivery, remarkable performance, and future-ready capabilities, the Navitor TAVI System sets a new standard in the field of TAVI, which is also known as TAVR (Transcatheter Aortic Valve Replacement).
WATCH TAVI SUMMIT:
WATCH THE LIVE CASES:
LIVE CASE FROM EACTS TECHNO-COLLEGE LIVE CASE FROM RIGSHOSPITALET COPENHAGEN
EXCELLENCE ELEVATED IN TAVI
The Navitor TAVI System is intuitive, precise, and accurate, offering excellent hemodynamics and durability for TAVI procedures. Its design ensures a seamless and optimized valve replacement experience, making it a preferred choice for clinicians and patients alike.
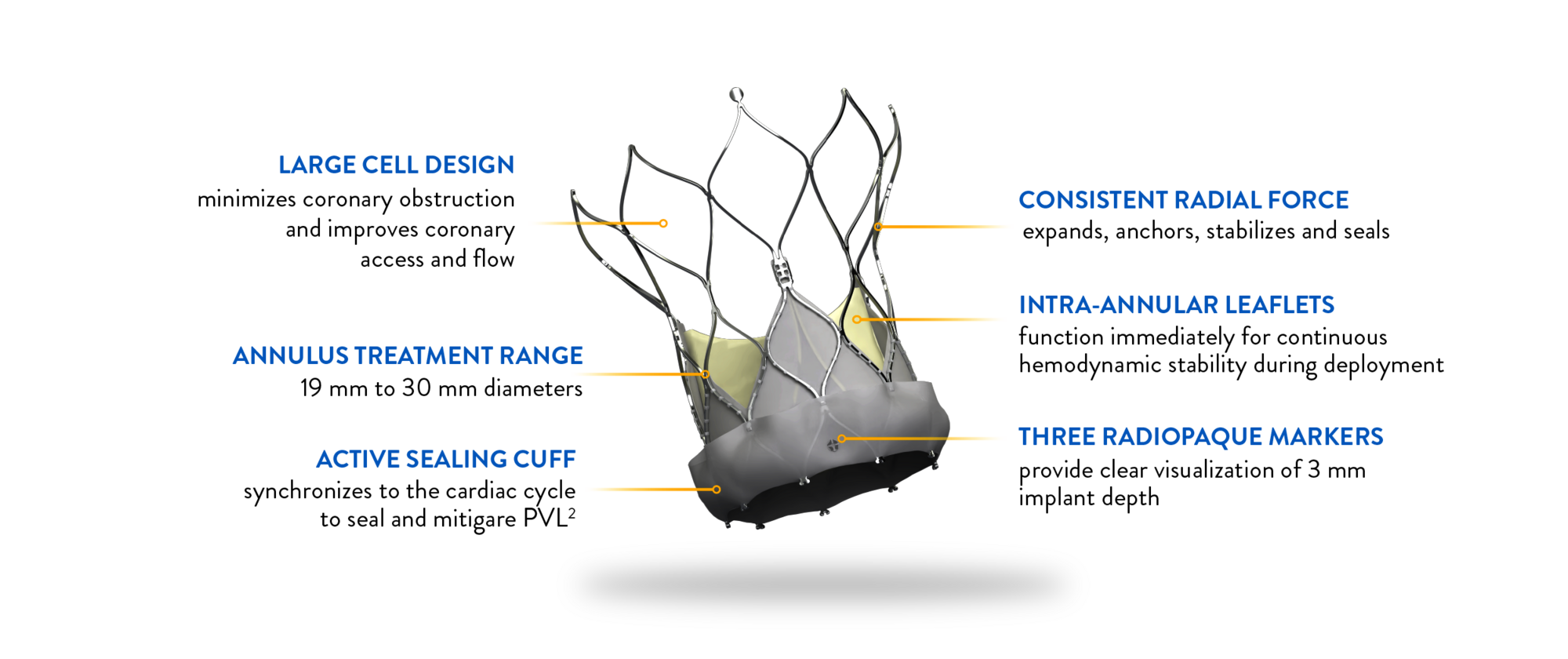
NAVITOR VISION* VALVE FEATURES
Attention to every detail
The Navitor Vision Valve is engineered with meticulous attention to detail, ensuring optimal outcomes for TAVI procedures.
EXCELLENT OUTCOMES IN CLINICAL TRIAL RESULTS | 30-DAY1,2

DISCOVER FEATURES AND BENEFITS
OF THE NAVITOR™ TAVI SYSTEM.
TV
TV
- Søndergaard L, Walton AS, Worthley SG, et al. Thirty-day and one-year outcomes of the Navitor transcatheter heart valve in patients with aortic stenosis: the prospective, multicentre, global PORTICO NG study. Eurointervention 2023;EIJ-D-22-01108. eurointervention.pcronline.com/article/thirty-day-and-one-year-outcomes-of-thenavitor-transcatheter-heart-valve-in-patients-with-aortic-stenosis-the-prospective-multicentre-global-portico-ng-study.
- Søndergaard, L. 30-day outcomes from a next generation TAVI device with an active sealing cuff. Presented at: EuroPCR conference; May 18–20, 2021.
- Pibarot P, Hahn RT, Weissman NJ, et al. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8(3):340–360.
doi.org/10.1016/j.jcmg.2015.01.008. - Frater RWM, Seifter E, Liao K, et al. Advances in Anticalcific and Antidegenerative Treatment of Heart Valve Bioprostheses. Austin, TX: Silent Partners Inc; 1997:105–114.
- Kelly SJ, Ogle, MF, Carlyle WC, et al. Biocompatibility and calcification of bioprosthetic heart valves. Society for biomaterials. Sixth World Biomaterials Congress Transaction. 2000;13534.
- Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation: efficacy and mechanisms. Circulation.
1997;95(2):479–488. doi.org/10.1161/01.CIR.95.2.479. - Vyavahare N, Hirsch D, Lerner E, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40(4):577–585.https://doi.org/10.1002/(SICI)1097-4636(19980615)40:4%3C577::AID-JBM9%3E3.0.CO;2-C.
- Gross JM. Calcification of bioprosthetic heart valves and its assessment. J Thorac Cardiovasc Surg. 2003;125:S6–8. doi.org/10.1067/mtc.2003.208.
- Tod TJ, Dove JS. The association of bound aldehyde content with bioprosthetic tissue calcification. J Mater Sci: Mater Med. 2016;27:8.
doi.org/10.1007/s10856-015-5623-z. - Meuris B, De Praetere H, Strasly M, et al. A novel tissue treatment to reduce mineralization of bovine pericardial heart valves. JTCVS. 2018;156(1):197–206. doi.org/10.1016/j.jtcvs.2018.01.099.
- Data on file at Abbott. 90664679
- Medtronic CoreValve Evolut‡ PRO Instructions for Use.
- Navitor™ TAVI System Instructions for Use.
- Boston Scientific ACURATE neo2‡ Instructions for Use.
- Boston Scientific iSleeve‡ Instructions for Use.
- Edwards Sapien‡ 3 Instructions for Use.
- Koehler Sapien‡ 3 eSheath OD BMRI 2015.
- Abbott data on file 90368819.
- Abbott data on file 90346620.
- Portico™ TAVI System Instructions for Use
‡Indicates a third-party trademark, which is the property of its respective owner.
Caution: These products are intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available)or at eifu.abbottvascular.com or at medical.abbott/manuals for more detailed information on Indications, Contraindications, Warnings, Precautions.
Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Abbott.