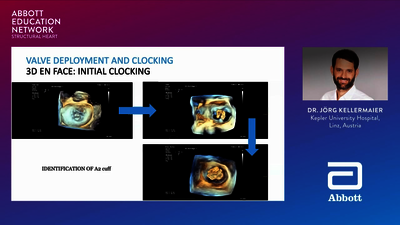
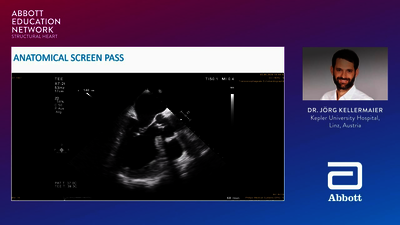
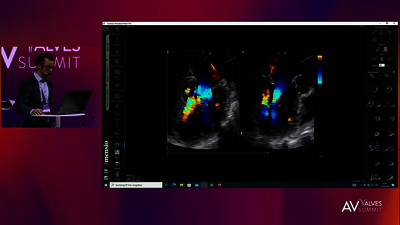
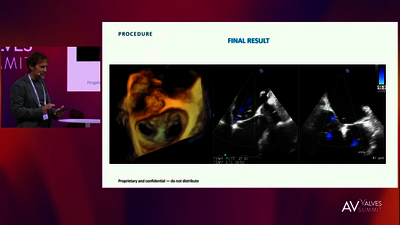
REPLACEMENT (TMVR)
Tendyne™ TMVR System is first-in-class technology designed to eliminate Mitral Regurgitation (MR),
offering selected patients with MR (≥ grade 3) a novel option for mitral valve replacement.
PATIENTS WHO BENEFIT
MR IS THE MOST COMMON VALVULAR HEART DISEASE AND IS SIGNIFICANTLY UNDERTREATED3
More patients are suffering from MR than any other valve disease, and only a small percentage of them are treated.2*† Significant MR is 4 times more prevalent than significant aortic stenosis.3,4

Solutions are needed beyond surgery
In one survey, almost 50% of symptomatic patients with severe MR were not candidates for mitral valve surgery due to underlying factors, including:5*
- Impaired left ventricle (LV) ejection fraction
- High operative risk
- Multiple comorbidities
- Advanced age
For these patients most in need of intervention, medical management is not enough.
Greater MR reduction is associated with higher survival
Real-world evidence shows a significant relationship between degree of residual MR and survival after transcatheter mitral repair.
Tendyne™ is an option for select MR patients who may benefit from MR elimination.6POST-PROCEDURAL MITRAL REGURGITATION6
FROM UNITED STATES TVT REGISTRY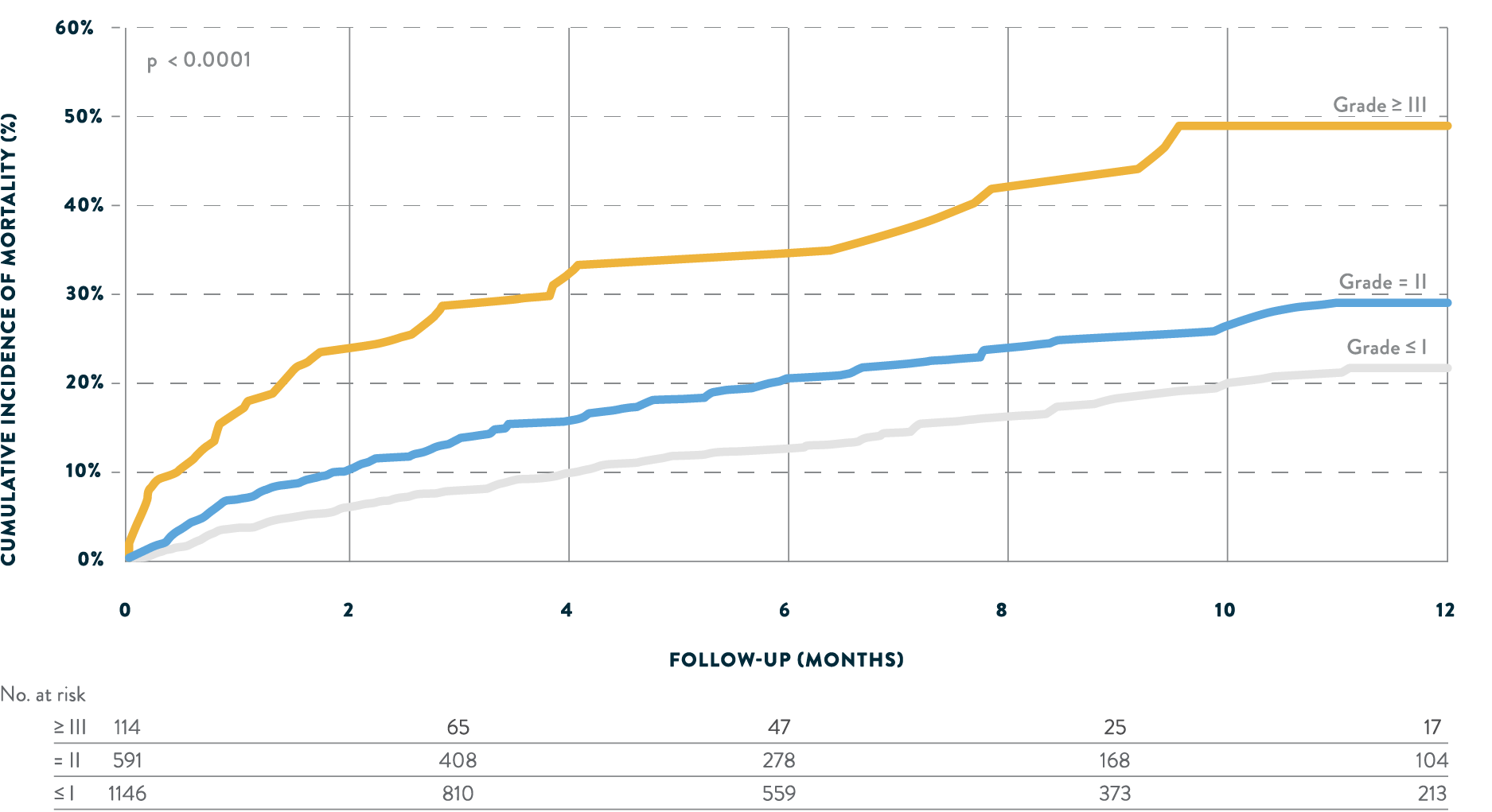
TENDYNE™ TMVR OFFERS PATIENTS WITH SEVERE MR (GRADE ≥3) A NEW OPTION FOR SAFE AND
EFFECTIVE MITRAL VALVE REPLACEMENT
When heart teams consider that conventional surgery or Transcatheter Edge-to-Edge Repair (TEER) is not indicated in patients with Primary or Secondary MR, TMVR therapy is the preferred option if the patient is deemed clinically and anatomically suitable.
Tendyne™ Mitral Valve System is indicated for the treatment of the native mitral valve without prior mitral valve intervention in patients with:
- Moderate-to-severe or severe mitral valve regurgitation (MR Grade ≥3+)
- A life expectancy less than 5 years
- Left ventricular end systolic diameter (LVESD) size >3 cm
- Left ventricular ejection fraction (LVEF) ≥ 30%
- Left ventricular end-diastolic dimension (LVEDD) ≤ 7.0 cm, who do not have severe mitral annular calcification and are deemed not suitable for surgical repair or replacement by a multi-disciplinary heart team.
Hub
TV
- Tendyne™ IFU.
- Data on file at Abbott.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011.
doi.org/10.1016/S0140-6736(06)69208-8. - United States Census Bureau, 2010. Age and sex composition. Issued May 2011:1–16.
- Mirabel M, Lung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28(11):1358–1365.
doi.org/10.1093/eurheartj/ehm001. - Sorajja P, Vemulapalli S, Feldman T, et al. Outcomes With Transcatheter Mitral Valve Repair in the United States: An STS/ACC TVT Registry Report. J Am Coll Cardiol. 2017;70(19):2315–27. doi.org/10.1016/j.jacc.2017.09.015.
- Sorajja P, Moat N, Badhwar V, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. 2019;73(11):1250–1260.
doi.org/10.1016/j.jacc.2018.12.066. - Gammie JS, Chikwe J, Badhwar V, et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg. 2018;106(3):716–27.
doi.org/10.1016/j.athoracsur.2018.03.086.