OCCLUDER
STRUCTURAL INTERVENTIONS
The primary treatment option for atrial septal defects (ASD) is percutaneous,
transcatheter closure, and Abbott’s Amplatzer™ Septal Occluder is the proven
standard of care2,5—with more than 20 years of global clinical experience in ASD closure.1
Two Amplatzer™ ASD Closure Devices for Optimizing Patient Care
Abbott offers two devices for percutaneous, transcatheter ASD closure.
Amplatzer™ Septal Occluder
The Amplatzer™ Septal Occluder, approved in the U.S. since 2001, is intended for patients with an ASD in the secundum position or patients who have undergone a fenestrated Fontan procedure and who now require closure of the fenestration.6
The Amplatzer Septal Occluder is designed for ASD closure:
- Shaped-memory nitinol mesh that securely apposes both sides of the septal wall
- A wide waist that centers the device and fills the ASD
- Polyester material that promotes occlusion and tissue in-growth
- Can be recaptured and redeployed for precise placement6
The Amplatzer Septal Occluder has the widest range of device sizes, from 4mm to 40mm, enabling the treatment of the widest range of ASD defects.
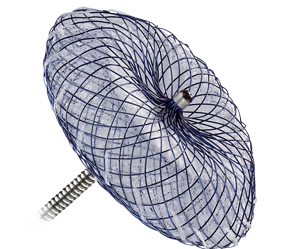
Amplatzer™ Cribriform Multi-Fenestrated Septal Occluder
The Amplatzer™ Cribriform Occluder is a percutaneous, transcatheter, ASD device intended for the closure of multifenestrated (cribriform) ASDs.10 Multiple, or fenestrated, ASDs that require closure are not uncommon. In fact, nearly 10% of patients with secundum-type ASD are found to have multifenestrated ASDs.7
In many ways similar to the Amplatzer Septal Occluder, the Amplatzer Cribriform design features include:
- Self-expanding, double-disc device designed for closure of multi-fenestrated ASDs
- Matched disc diameters that maximize coverage of multiple fenestrations
- Ability to be easily recaptured and redeployed for optimal placement1
The Amplatzer Cribriform Occluder disc diameters range from 18 mm to 40 mm.

TV