TAVI SYSTEM
TAVI
Stable delivery. Remarkable performance. Future ready.
NAVITOR™ TAVI SYSTEM
Navitor™ combines Navitor™ valves (available in four sizes) with the FlexNav™ delivery system to provide one TAVI system, optimized to give favorable clinical outcomes while maintaining enhanced deliverability and ease of use.
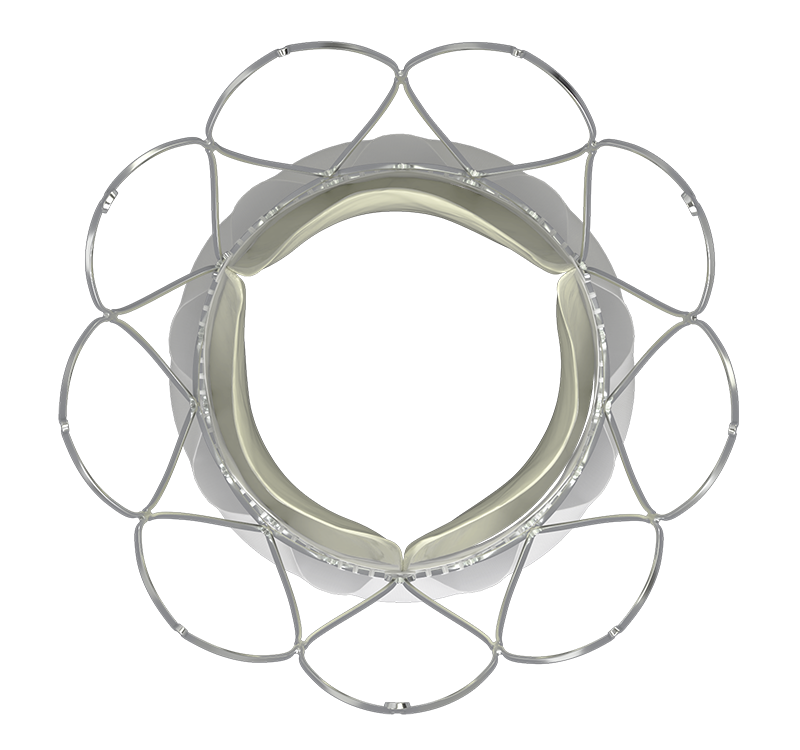
NAVITOR™ VALVE FEATURES
INTELLIGENT DESIGN
Advancing the forefront of innovative design, the Navitor™ valve brings together smart PVL-sealing technology, exceptional single-digit gradients,1,2 and uncompromised coronary access to achieve excellent clinical outcomes.
SMART SEALING.
REMARKABLE PERFORMANCE.
NaviSeal™ Cuff actively synchronizes to the cardiac cycle, seals, and mitigates paravalvular leak (PVL)1,2 by expanding to fill calcification-related gaps between the annulus and the valve.
SMART SEALING MITIGATES PVL | 30-DAY ECHO CORE LAB DATA1,2

2.0 cm2
EOA
7.4 mmHg
Mean gradient
HEMODYNAMIC IMPACT
Non-tapered stent and large effective orifice areas resulting in single-digit gradients are associated with improved cardiac function, long-term durability, and minimal prosthesis-patient mismatch.1,2

Continuous stability.
No rapid pacing.
The only self-expanding valve with intra-annular leaflets that immediately function and a nontapered stent, providing hemodynamic stability for a calm and controlled deployment.

Designed for durability.
Exclusive Linx™ anticalcification (AC) technology resists calcification in four distinct ways to improve long-term valve performance.4-7
| ABBOTT LINX™ AC*4-7 | MEDTRONIC AOA‡8* | BOSTON SCIENTIFIC BIOFIX‡* | EDWARDS THERMAFIX‡9,10* | |
|---|---|---|---|---|
| PRODUCTS | NAVITOR™ | EVOLUT‡ PRO | ACURATE NEO2‡ | SAPIEN‡ 3 |
| Reduces free aldehydes | ✔ | ✔ | Not Publicly Available | ✔ |
| Extracts lipids | ✔ | Not Publicly Available | ✔ | |
| Minimizes uptake of cholesterol | ✔ | Not Publicly Available | ||
| Stabilizes leaflet collagen | ✔ | Not Publicly Available |


Large-cell geometry and intra-annular valve design preserve coronary access for future intervention.
| VALVE SIZE | NAVITOR™*11 | EVOLUT‡ PRO*12 |
|---|---|---|
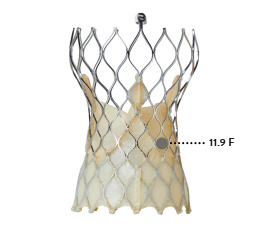
| 23 mm | 14.6 F | 12.1 F |
| 25 mm | 16.3 F | n/a |
| 26 mm | n/a | 11.8 F |
| 27 mm | 18.7 F | n/a |
| 29 mm | 21.0 F | 11.9 F |

36 cells total
9 cells in the annulus
section of the stent
135 cells total
15 cells in the annulus
section of the stent
FLEXNAV™ DELIVERY SYSTEM FEATURES
STABILITY AND ACCURACY
- Low profile and highly flexible catheter enables excellent deliverability,
even in patients with small access vessels and tortuous anatomies - Controlled deployment provides stable and accurate valve placement
- Recapturable,* repositionable,* and retrievable* design
14 F† DELIVERY SYSTEM
WITH 5.0 mm MINIMUM
VESSEL DIAMETER
FLEXNAV™ DELIVERY SYSTEM FEATURES
STABILITY AND ACCURACY
- Low profile and highly flexible catheter enables excellent deliverability,
even in patients with small access vessels and tortuous anatomies - Controlled deployment provides stable and accurate valve placement
- Recapturable,* repositionable,* and retrievable* design
14 F† DELIVERY SYSTEM
WITH 5.0 mm MINIMUM
VESSEL DIAMETER
| NAVITOR WITH FLEXNAV™13 | EVOLUT‡ PRO WITH ENVEO‡ PRO12 | ACURATE NEO2‡ WITH ISLEEVE‡14,15 | SAPIEN‡ 3 WITH ESHEATH‡16,17 | |
|---|---|---|---|---|
| Delivery System Profile (Outer Diameter) | 6.0 mm 6.3 mm | 6.7 mm | 6.0 mm | 7.6 mm 8.2 mm |
| Minimum Vessel Diameter | 5.0 mm 5.5 mm | 5.5 mm | 5.5 mm | 5.5 mm 6.0 mm |
See progress as you go
Feel every advancement
Open and close with ease
TAKE THE TENSION OUT OF TAVI
Lock in placement accuracy
Dial in the details
A deployment indicator gives you a clear visualization of the valve’s deployment progress.
The simple deployment wheel provides both audible and tactile feedback as you deploy the valve.
Two macro-slide buttons make it easy for you to open and close the distal end of the delivery system during valve loading and post-deployment.
The stability layer ensures stable, predictable valve deployment to achieve accurate valve placement.
The automatic lock button prevents full valve deployment until you’ve got the valve right where you want it.
With the unique micro-adjustment wheel, you can close gaps between the valve capsule and the atraumatic nosecone.

See progress as you go
A deployment indicator gives you a clear visualization of the valve’s deployment progress.Feel every advancement
The simple deployment wheel provides both audible and tactile feedback as you deploy the valve.Open and close with ease
Two macro-slide buttons make it easy for you to open and close the distal end of the delivery system during valve loading and post-deployment.TAKE THE TENSION OUT OF TAVI
The stability layer ensures stable, predictable valve deployment to achieve accurate valve placement.Lock in placement accuracy
The automatic lock button prevents full valve deployment until you’ve got the valve right where you want it.Dial in the details
With the unique micro-adjustment wheel, you can close gaps between the valve capsule and the atraumatic nosecone.FlexNav™ Delivery System offers three-dimensional flexibility at the distal end and throughout its entire working length.
Atraumatic nosecone
Large-Cell framework
Flexible capsule
Hydrophilic coating
Integrated sheath
Atraumatic nosecone and smooth transitions are designed to
reduce risk of vascular complications and calcium dislodgement.The large-cell framework of the NavitorTM valve reduces metal mass, resulting in a more flexible capsule.
The capsule is more flexible because it does not require bilateral metal rails or extra nitinol braiding, resulting in enhanced flexibility.18
Hydrophilic coating reduces friction by 98%,19 providing lubricity to guide the system through vasculature.
Integrated sheath for low 14 F delivery profile.20*
* 14 F equivalent integrated sheath diameter for patients requiring 23 mm or 25 mm valve.

- Atraumatic nosecone and smooth transitions are designed to reduce risk of vascular complications and calcium dislodgement.
- The large-cell framework of the NavitorTM valve reduces metal mass, resulting in a more flexible capsule.
- The capsule is more flexible because it does not require bilateral metal rails or extra nitinol braiding, resulting in enhanced flexibility.17
- Hydrophilic coating reduces friction by 98%,18 providing lubricity to guide the system through vasculature.
- Integrated sheath for low 14 F delivery profile.19*
* 14 F equivalent integrated sheath diameter for patients requiring 23 mm or 25 mm valve.
In terms of delivery of the valve, the FlexNav™ is now a leader by far. It has the best access for sheathless approach… It cuts through like (a) hot knife through butter.
Interventional Cardiologist, Ireland*
Connext
Live
TV
- Søndergaard L, Walton AS, Worthley SG, et al. Thirty-day and one-year outcomes of the Navitor transcatheter heart valve in patients with aortic stenosis: the prospective, multicentre, global PORTICO NG study. Eurointervention 2023;EIJ-D-22-01108. eurointervention.pcronline.com/article/thirty-day-and-one-year-outcomes-of-thenavitor-transcatheter-heart-valve-in-patients-with-aortic-stenosis-the-prospective-multicentre-global-portico-ng-study.
- Søndergaard, L. 30-day outcomes from a next generation TAVI device with an active sealing cuff. Presented at: EuroPCR conference; May 18–20, 2021.
- Pibarot P, Hahn RT, Weissman NJ, et al. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8(3):340–360. doi.org/10.1016/j.jcmg.2015.01.008.
- Frater RWM, Seifter E, Liao K, et al. Advances in Anticalcific and Antidegenerative Treatment of Heart Valve Bioprostheses. Austin, TX: Silent Partners Inc; 1997:105–114.
- Kelly SJ, Ogle, MF, Carlyle WC, et al. Biocompatibility and calcification of bioprosthetic heart valves. Society for biomaterials. Sixth World Biomaterials Congress Transaction. 2000;13534.
- Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation: efficacy and mechanisms. Circulation.
1997;95(2):479–488. doi.org/10.1161/01.CIR.95.2.479. - Vyavahare N, Hirsch D, Lerner E, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40(4):577–585.https://doi.org/10.1002/(SICI)1097-4636(19980615)40:4%3C577::AID-JBM9%3E3.0.CO;2-C.
- Gross JM. Calcification of bioprosthetic heart valves and its assessment. J Thorac Cardiovasc Surg. 2003;125:S6–8. doi.org/10.1067/mtc.2003.208.
- Tod TJ, Dove JS. The association of bound aldehyde content with bioprosthetic tissue calcification. J Mater Sci: Mater Med. 2016;27:8.
doi.org/10.1007/s10856-015-5623-z. - Meuris B, De Praetere H, Strasly M, et al. A novel tissue treatment to reduce mineralization of bovine pericardial heart valves. JTCVS. 2018;156(1):197–206. doi.org/10.1016/j.jtcvs.2018.01.099.
- Data on file at Abbott. 90664679
- Medtronic CoreValve Evolut‡ PRO Instructions for Use.
- Navitor™ TAVI System Instruct ions for Use.
- Boston Scientific ACURATE neo2‡ Instructions for Use.
- Boston Scientific iSleeve‡ Instructions for Use.
- Edwards Sapien‡ 3 Instructions for Use.
- Koehler Sapien‡ 3 eSheath OD BMRI 2015.
- Abbott data on file 90368819.
- Abbott data on file 90346620.
- Portico™ TAVI System Instructions for Use
‡Indicates a third-party trademark, which is the property of its respective owner.