VALVE SOLUTIONSEPIC™ PLUS MITRAL AND AORTIC STENTED TISSUE VALVES
The latest generation of the Epic™ Platform continues the Epic product family’s history of excellence. The future-forward design of the Epic Plus mitral and aortic valves gives you and your patients more choices when it comes to preserving long-term treatment paths.
THE PLATFORM FOR POSSIBILITY
The Epic™ Platform has been proven over time and defined by strong hemodynamics, intuitive implantability, a future-forward design, and more. The platform boasts the low ventricular protrusion in all valve sizes, a flexible polymer stent, individually selected porcine leaflets to ensure optimal coaptation, and a flexible sewing cuff designed to minimize both in-implant suture drag and post-implant paravalvular leak.
- Silicone-Filled Cuff
- FlexFitTM Polymer Stent
- Unique Pericardial Shield
- Flexible Cuff
- Low Stent Post Height
Allows for supra-annular implantation
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
Mitigates PVL and easily fits patient anatomy
Mitigates risk of coronary obstruction
EPIC™ SUPRA AORTIC STENTED TISSUE VALVE FEATURES

SILICONE-FILLED CUFF
Allows for supra-annular implantation
FLEXFIT™ POLYMER STENT
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
UNIQUE PERICARDIAL SHIELD
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
FLEXIBLE CUFF
Mitigates PVL and easily fits patient anatomy
LOW STENT POST HEIGHT
Mitigates risk of coronary obstruction
- Optimal Leaflet Design and Matching
- Low Ventricular Protrusion
- FlexFitTM Polymer Stent
- Unique Pericardial Shield
- Flexible Cuff
- Ratcheting Holder
Reduces regurgitation risk
Avoids LVOT obstruction
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
Mitigates PVL and easily fits patient anatomy
Streamlines implant and reduces risk of suture looping
EPIC™ MITRAL STENTED TISSUE VALVE FEATURES
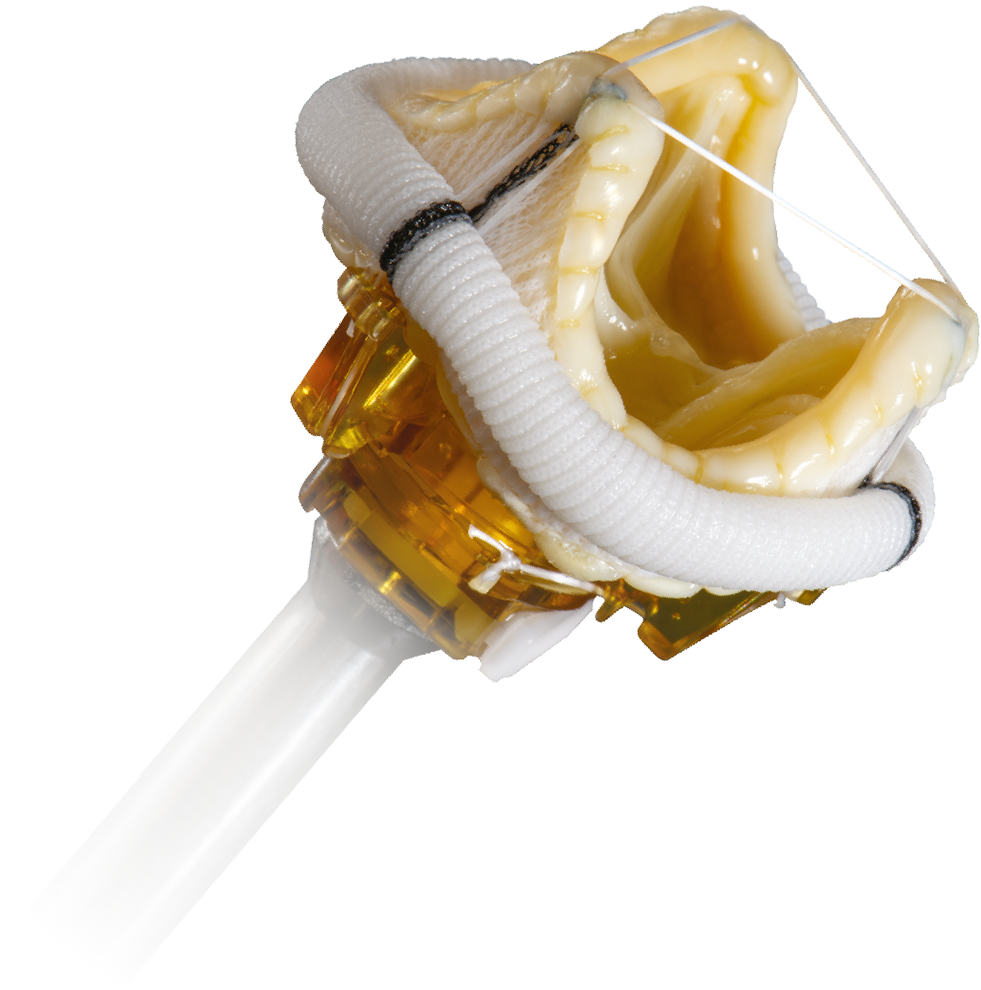
OPTIMAL LEAFLET DESIGN AND MATCHING
Reduces regurgitation risk
LOW VENTRICULAR PROTRUSION
Avoids LVOT obstruction
FLEXFIT™ POLYMER STENT
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
UNIQUE PERICARDIAL SHIELD
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
FLEXIBLE CUFF
Mitigates PVL and easily fits patient anatomy
RATCHETING HOLDER
Streamlines implant, reduces risk of suture looping, and offers true one cut holder release
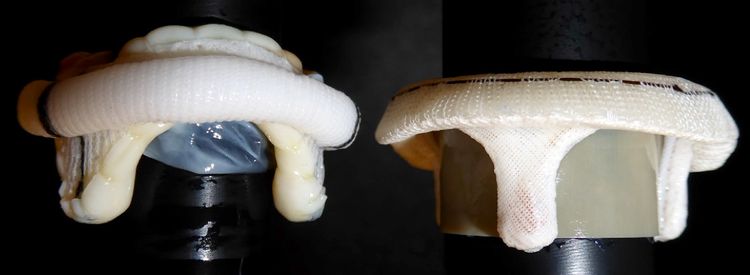
Curtaining is a characteristic of bioprosthetic valves in which the leaflets, when opened, stand tall or form a “curtain” between stent posts.10,* Epic/Epic Plus Mitral leaflets are not prone to curtaining, which can result in less LVOT obstruction.10,*,†
Leaflet behavior when opened
Abbott’s unique Linx™ AC technology—to promote anticalcification*—is designed to improve long-term performance and valve durability.
The Linx™ AC treatment has demonstrated resistance to calcification by:
- Extracting lipids6
- Reducing free aldehydes7,8
- Minimizing cholesterol uptake9
- Stabilizing leaflet collagen9
Clinical Case clubs | Educational tools | Hot topics
Expert opinions | Live & online discussions
TV
- Guler N, Ozkara C, Akyol A. Left ventricular outflow tract obstruction after bioprosthetic mitral valve replacement with posterior mitral leaflet preservation. Tex Heart Inst J. 2006;33(3):399-401.
- Epic Plus Instructions for Use.
- Allen KB, Adnan CK, Cohen DJ, et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. 2017;104:1501-1508.
- Edelmann JJ, Khan JM, Rogers T, et al. Valve-in-valve TAVR: state-of-the-art review. Innovations. 2019;14(4):299-310.
- Jawad, Khalil, Sven Lehmann, Alex Koziarz, Maja Dieterlen, Stefan Feder, Martin Misfeld, Jens Garbade, Vivek Rao, and Michael Borger. "Midterm results after St Jude Medical Epic porcine xenograft for aortic, mitral, and double valve replacement." Journal of Cardiac Surgery 35, no. 8 (2020): 1769-1777.
- Adams DH, Rosenhek R, and Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31(16):1958-1967. doi:10.1093/eurheartj/ehq222.
- Otto, Catherine M., Rick A. Nishimura, Robert O. Bonow, Blase A. Carabello, John P. Erwin III, Federico Gentile, Hani Jneid et al. "2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines." Journal of the American College of Cardiology 77, no. 4 (2021): 450-500.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation. 2014;129(23):2240-2492.
- Mick SL, Keshavamurthy S, Gillinov AM. Mitral valve repair vs replacement for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
- Tests performed by and data on file at Abbott.
- Magna Mitral Ease Instructions for Use.
- Medtronic Mosaic Bioprosthesis Aortic and Mitral Brochure. #UC200103933b EN.
- Vyavahare, N, Hirsch, D, Lerner, E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Circulation. 1997:95;479-488. doi.org/10.1161/01.CIR.95.2.479.
- Kelly SJ, Ogle MF, Carlyle WC, et al. Biocompatibility and calcification of bioprosthetic heart valves. Presented at: Society for Biomaterials, Sixth World Biomaterials Congress Transaction. May 2000:1353.
- Frater RWM, Seifter E, Liao K, et al. Chapter 8. In: Gabbay, S, Wheatley DJ, eds. Advances in Anticalcific and Antidegenerative Treatment of Heart Valve Bioprostheses. 1st ed. Silent Partners, Inc; 1997:105-113.
- Vyavahare NR, Hirsch D, Lerner E, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40:577-585. doi.org/10.1177/1556984519858020.



